Describe the Nucleus of an Atom
The radius of an atom is about 01 nm 1 10-10 m the. Nucleus contains protons which have a positive charge equal in magnitude to the electrons negative charge.

Components Of An Atomwhat Makes Up An Atom Orbiting Around The Nucleus Are The Electrons Electrons Atom Atomic Structure Middle School Science
Physical Science - Chapter 4 Test Review Vocab 61 terms.
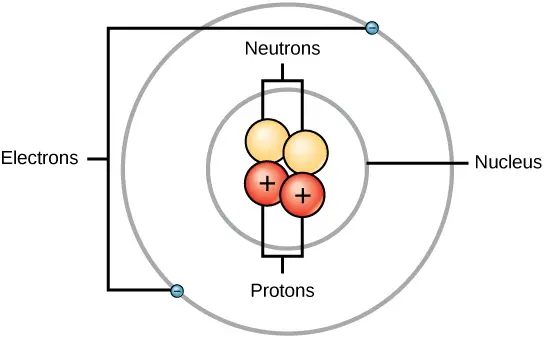
. The quantity b. And at the center of an atom is known as nucleus in which composed of protons and neutrons. AThe nucleus is the dense center of the atom composed of protons and neutrons.
This is surrounded by electrons arranged in shells. Now positive charge on the nucleus is due to the presence of protons in it and each proton carries one unit of positive charge. Empty space or vacuum.
The nucleus of ordinary hydrogen contains a single proton. The number of protons present in the nucleus of an atom of an element is known as its atomic number. The atomic number of Al atom is 13 which means that there is 13 protons in the nucleus.
The protons are positively charged which gives the nucleus a positive charge while the neutrons have no charge. The nucleus is orbited by the atoms electrons. So the nucleus has a charge of 13.
The answer is 2. Electrons are organized into shells which is a region where an electron is most likely found. The attractions between the shred electrons and protons in the nucleus hold the atom together in a covelant bond.
The nucleus of an atom is a small dense region at the center of an atom that contains protons and neutrons. 5 Which statement best describes the structure of an atom. 3 ρ r ρ o 1 erR0b In this case the effective radius R of the nucleus is equal to R0 b.
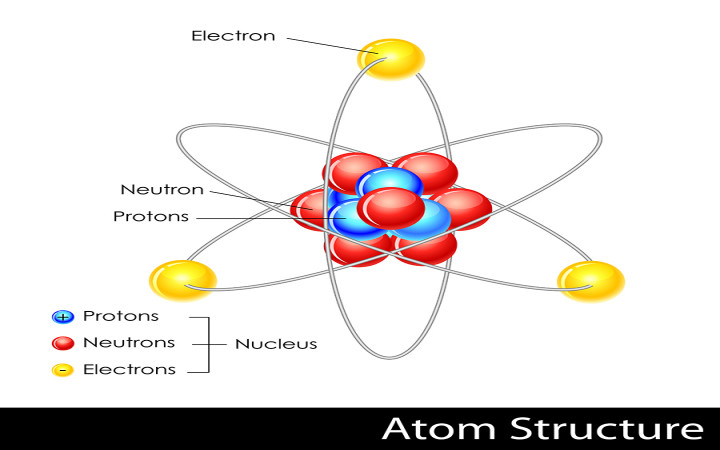
The nucleus of an atom is made up of protons and neutrons. As you may recall the two particles that are found in the nucleus are the protons and neutrons. The atomic number is denoted by Z.
The average mass of an atom taking into account all its naturally occurring isotopes. They describe the type and number of atoms in a molecule of the compound. The nucleus of an atom contains protons and neutrons.
The forces inside the nucleus of a stable atom are balanced because the nucleus contains the proper number of protons and neutrons. Surrounding the nucleus is an electron cloud composed of electrons. The nucleus carries a positive electrical charge.
Surrounding the nucleus is. An atom of carbon has 6 protons so the atomic number of carbon is 6. Describe the structure of an atom.
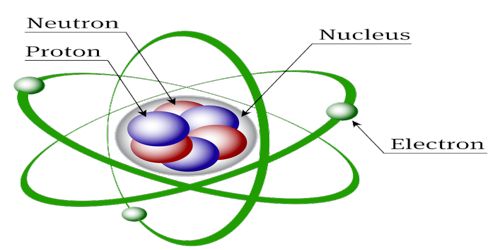
The atom consists of a tiny nucleus surrounded by moving electrons. Where μ is the mass of the meson h is Plancks constant and c is the speed of light in a vacuum. Atom is the smallest unit of a chemical element.
The nucleus is tiny compared to the atom as a whole. So there is 13 electrons surrounded the nucleus. These two units are collectively called nucleons.
Describe the structure of the atom. What does the electron cloud model describe. All atoms are made up of the nucleus and electrons.
These atoms are theoretically resistant to all forms of decay except proton decay which is a hypothetical form of decay that has never been observed in a. A The nucleus is the dense center of the atom composed of protons and neutrons. What is between electron and nucleus.
Almost all the mass of an atom is located in the nucleus with a very small contribution from the electron shells. An atom has a central nucleus surrounded by electrons arranged in areas called shells. 1 r0 ℏμc.
And the Al atom has net charge of 0. 2 protons and 2 neutrons are lost. The sum of the number of protons and the number of neutrons in an atom.
The structure of a carbon atom not drawn to scale The nucleus is very small compared to the atom as a. The nucleus is the control center of the entire atom the nucleus of an atom has 3 basic subatomic particles. -the electrons which orbit the outside of the nucleus electrons have a negative charge.
6 Why is it important to know the structure of an atom. When two atoms share one pair of valence electrons. Forces produced by the exchange of.
8 Which statement best explains why the overall charge on an atom is zero quizlet. 1 Sub-atomic particles such as electrons protons and neutrons need to be treated as quantum objects. Moving around this proton is a single electron.
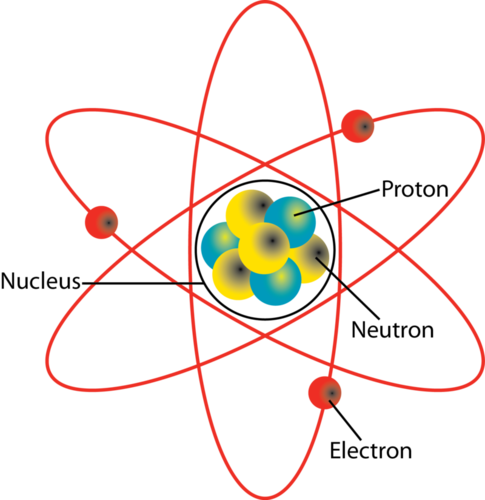
The nucleus may also contain neutrons which have virtually the same mass but no charge. Location where an electron has the highest probability of being found What is the atomic number of an oxygen atom with 8 protons and 10 neutrons in its nucleus. An atom has a central nucleus.
Because the atomic number of an element is the number of protons its atom has a. Electrons move around outside the nucleus. Mass number decreases by 4 Atomic number decreases by 2 Beta decay 1 neutron is converted to an electron lost from the atom and proton Mass number is unchanged Atomic number increases by 1 Gamma decay Energy is lost from an atom in the form of.
7 Which statement explains why most of the mass of an atom is contained in the small nucleus. Sets with similar terms. Thats the simple answer but there are a few subtleties.
The empty space between the atomic cloud of an atom and its nucleus is just that. 4 What defines the mass of an atom. The number of protons in an atom.
Describe the structure of an atom. The Atomic Nucleus The simplest possible atom and the most common one in the Sun and stars is hydrogen.
What Is Inside An Atom Wonderopolis
Structure Of The Nucleus In Atom Qs Study
What Two Particles Are Found In The Nucleus Of An Atom Socratic

Nucleus Definition Structure Function Cellular Vs Atomic Nuclei

Atom Rutherford S Nuclear Model Britannica

How To Describe A Nucleus And Its Functions Quora

Does Every Atom Have A Nucleus Quora
What Is The Nucleus Of Atom Qs Study
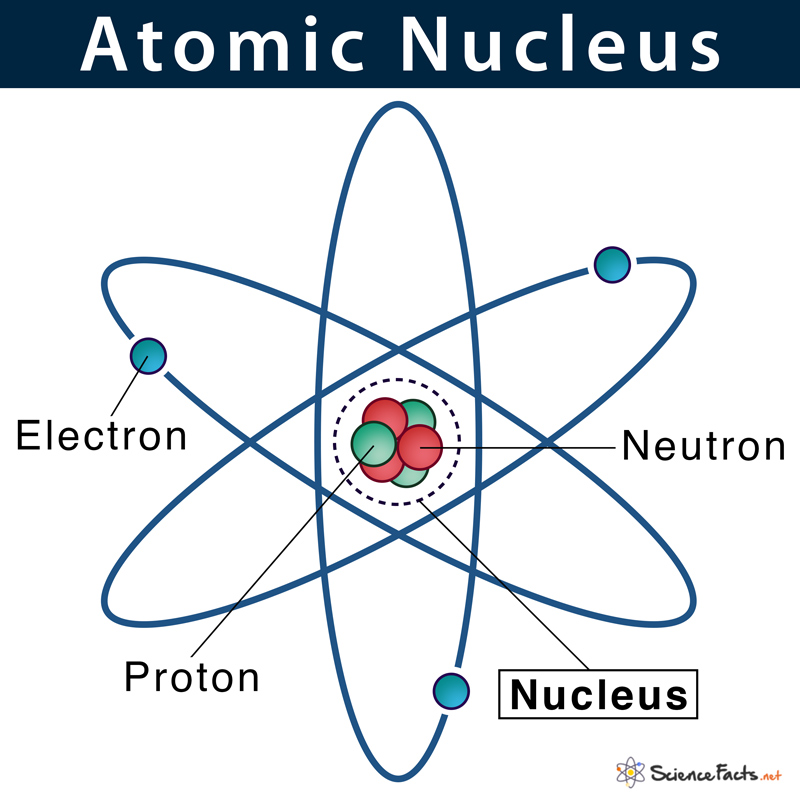
Atomic Nucleus Definition Structure Parts With Diagram
Atomic Nucleus Chemistry Encyclopedia Structure Reaction Elements Examples Gas Number Name Property

The Structure Of The Atom Astronomy

Nucleus Of The Atom Ck 12 Foundation
What Is An Atom And Atomic Nucleus Quora

What Is True Of An Atom S Nucleus Lisbdnet Com

Basic Parts Of The Atom Protons Neutrons Electrons Nucleus Youtube
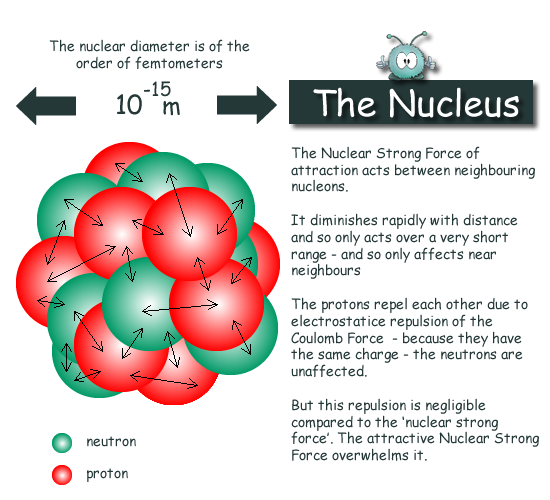
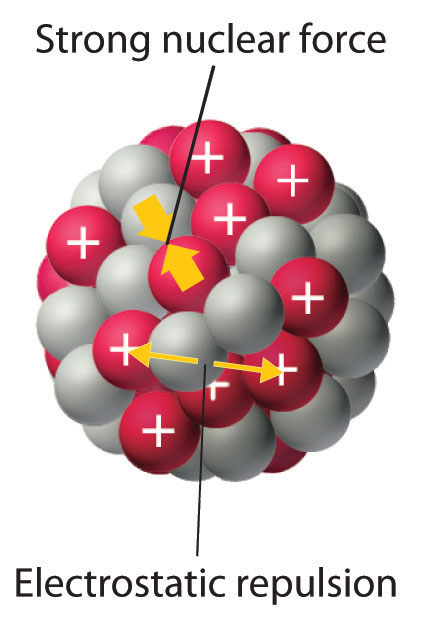

Comments
Post a Comment