What Does a Negative Hf for a Molecule Mean
When a molecule contains more than one bond the geometry must be taken into account. Between molecules of HF there is the dispersion force that is present between all molecules there is the dipole-dipole interaction between the positive end of the dipole the H end in HF and the negative end of the dipole the F end in HF.
The Geometries Of The Hf Hf Bh 3 Nh 3 Nacl Co 2 Co 2 And Download Scientific Diagram
For HF there is a larger dipole moment because there is a larger difference in electronegativity.

. Does a more negative heat of formation a larger negative number mean that a compound is more stable or less stable than an isomer with a less negative heat of formation. A polar molecule is a molecule in which one end of the molecule is slightly positive while the other end is slightly negative. If the bonds in a molecule are arranged such that their bond moments cancel vector sum equals zero then the molecule is nonpolar.
Polar means having electrical poles. Its danger lies in the fact that it can be absorbed by the skin causing burns which are not immediately noticed hence the danger. What does negative Hf for a molecule mean.
What does negative Hf for a molecule mean. Energy was added when the molecule was formed from its elements. 1 on a question What does a negative A Hf for a molecule mean.
Polar molecule definition a molecule in which the centroid of the positive charges is different from the centroid of the negative charges. Draw an energy diagram that illustrates this. Energy was released when the molecule was formed from its elements apex.
Energy was released when the molecule was formed from its elements apex. When solutes are added to. HFg is a colourless gas whose aqueous solution is known as Hydrofluoric acid which is the real danger.
When the product has. That means the reaction is endothermic. Energy was released when the molecule was formed from its elements apex.
Between molecules of HF there is also the special dipole-dipole interaction called hydrogen bonding. Examples of homonuclear nonpolar molecules are oxygen O 2 nitrogen N 2 and ozone O 3. Energy was released when the molecule was formed from its elements Goat.
2 See answers Advertisement Advertisement Kanekikenkun Kanekikenkun Energy was released when the molecule went through a phase change. What does a negative A Hf for a molecule mean. HF aq is a weird acid as it is classified as weak yet it is extremely dangerous.
Answer 1 of 15. This is an example of polar covalent chemical bonding. What does negative Hf for a molecule mean.
A diatomic molecule that consists of a polar covalent bond such as HF is a polar molecule. Chemistry questions and answers. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule.
The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. Water H 2 O is polar because of the bent shape of the molecule. Examples of polar molecules are Wat.
If compound A reacts with compound B to yield compound P and the transition state for the rate limiting step is A---B you calculate the energy of the reactants GM and the transition state A. Most carbon compounds are nonpolar. The molecules in which the arrangement or geometry of the atoms is such that one end of the molecule has a positive electrical charge and the other side has a negative charge are called as polar molecules.
This is an example of polar covalent chemical bonding. Nonpolar Molecule Examples. When the product has a greater enthalpy than the reactant then H will be positive.
Water H 2 O is polar because of the bent shape of the molecule. A diatomic molecule that consists of a polar covalent bond such as HF is a polar molecule. The two electrically charged regions on either end of the molecule are called poles similar to a magnet having a north and a south pole.
What does a negative A Hf for a molecule mean. Polarity of a Water Molecule. In progress 0 Chemistry Orla Orla 5 months 2021-08-21T2331500000 2021-08-21T2331500000 2 Answers 13 views 0.
A notable exception is carbon monoxide CO. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Other nonpolar molecules include carbon dioxide CO 2 and the organic molecules methane CH 4 toluene and gasoline.

The Geometries Of The Hf Hf Bh 3 Nh 3 Nacl Co 2 Co 2 And Download Scientific Diagram
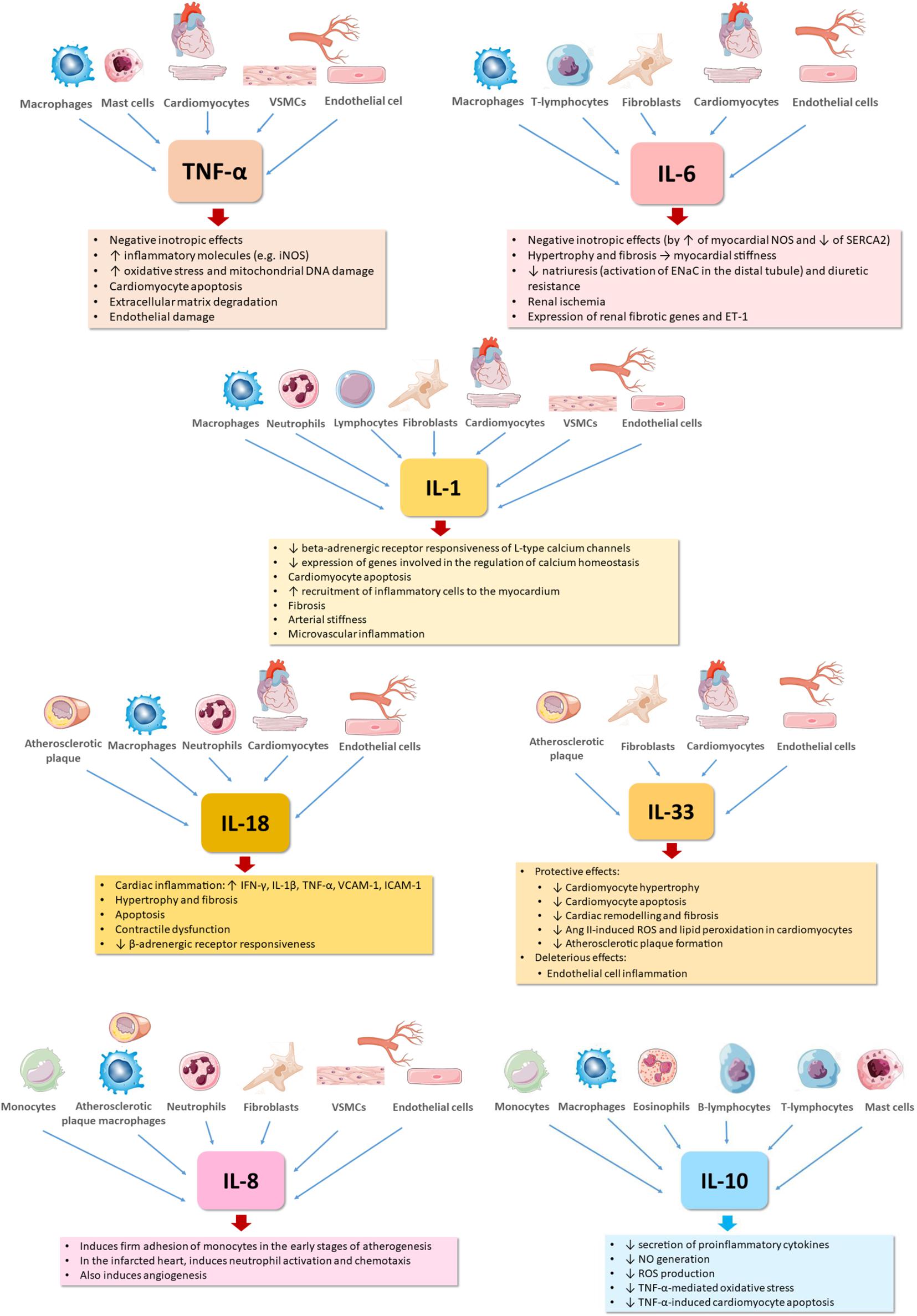
Frontiers Inflammation In Human Heart Failure Major Mediators And Therapeutic Targets Physiology
Molecules September 2018 Browse Articles

Molecules January 2017 Browse Articles

The Dipole Moment Of Hf Molecule Is 1 91d And The Bond Distance Is 0 92 A What Is The Fractional Charge On H And F In The Hf Bond Electronic Charge 4 8 Xx 10 10 E S U

Periodic Law Easy Science Science Flashcards Physical And Chemical Properties Periodic Table

Science Coverage Is No2 Polar Or Nonpolar Electron Affinity Covalent Bonding Molecular Geometry

Science Coverage Is Hi Polar Or Nonpolar Electron Affinity Covalent Bonding Redox Reactions

The Geometries Of The Hf Hf Bh 3 Nh 3 Nacl Co 2 Co 2 And Download Scientific Diagram

Covalent Bonding Worksheet Teaching Resources Covalent Bonding Worksheet Covalent Bonding Ionic And Covalent Bonds

Correlationenergies Maple Help

Science Coverage Free Solved In Each Reaction Box Place The Bes Solving Reaction Types Reactions

Is Hf Hydrogen Fluoride Polar Or Nonpolar
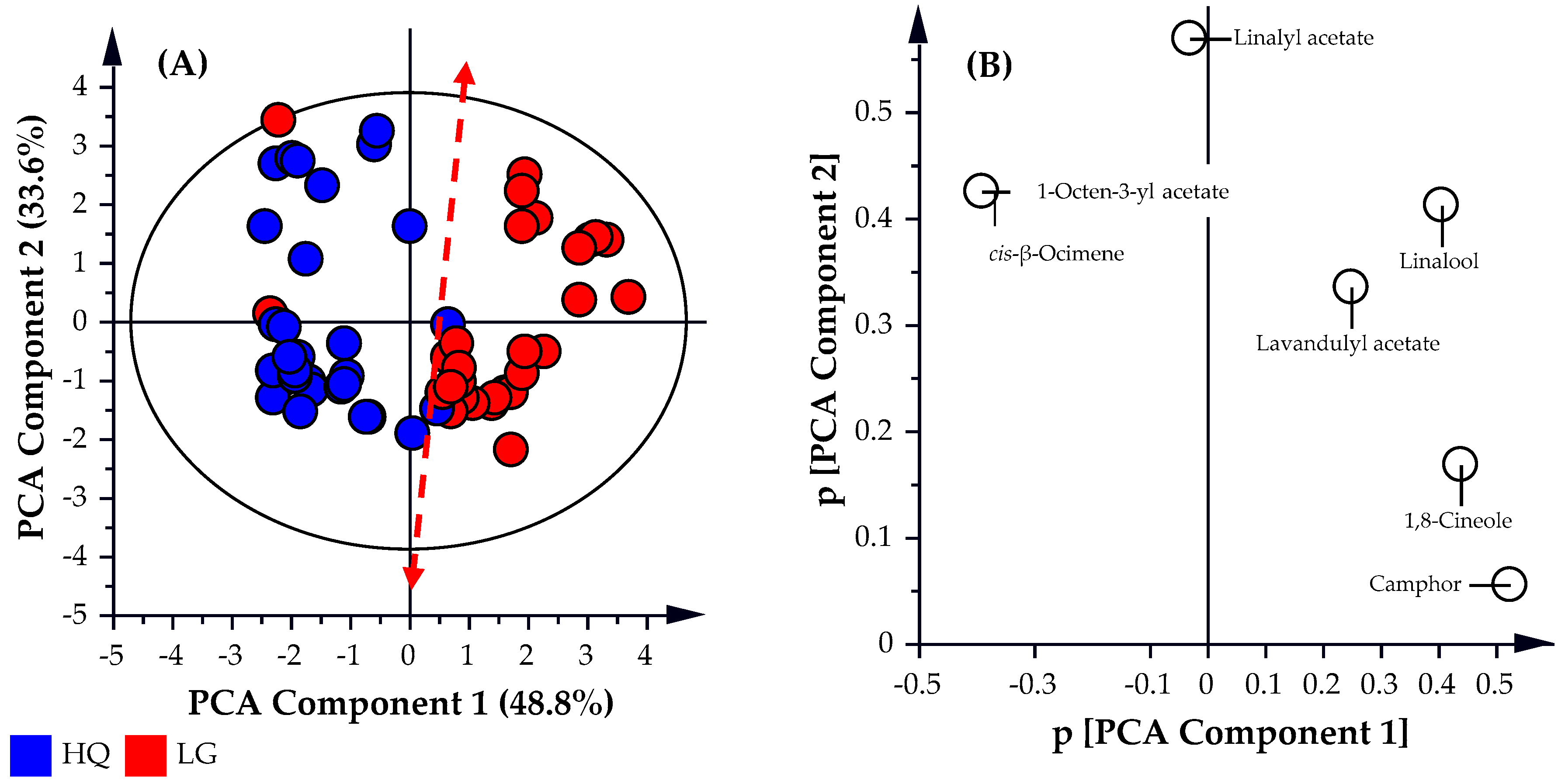
Molecules Free Full Text Chemometric Analysis Of Lavender Essential Oils Using Targeted And Untargeted Gc Ms Acquired Data For The Rapid Identification And Characterization Of Oil Quality Html

Premiere Suite Bali Nusa Dua Hotel

How To Draw Hf Lewis Structure Science Education And Tutorials

Hf Molecular Geometry Science Education And Tutorials

Jcs Jhfs 2021 Guideline Focused Update On Diagnosis And Treatment Of Acute And Chronic Heart Failure Journal Of Cardiac Failure

5 1 Delta Hf And Delta Hc Calculations Sl Ib Chemistry Youtube
Comments
Post a Comment